SkinMedica®
SkinMedica® is a physician-dispensed, cosmetic, and non-prescription skin care product line.
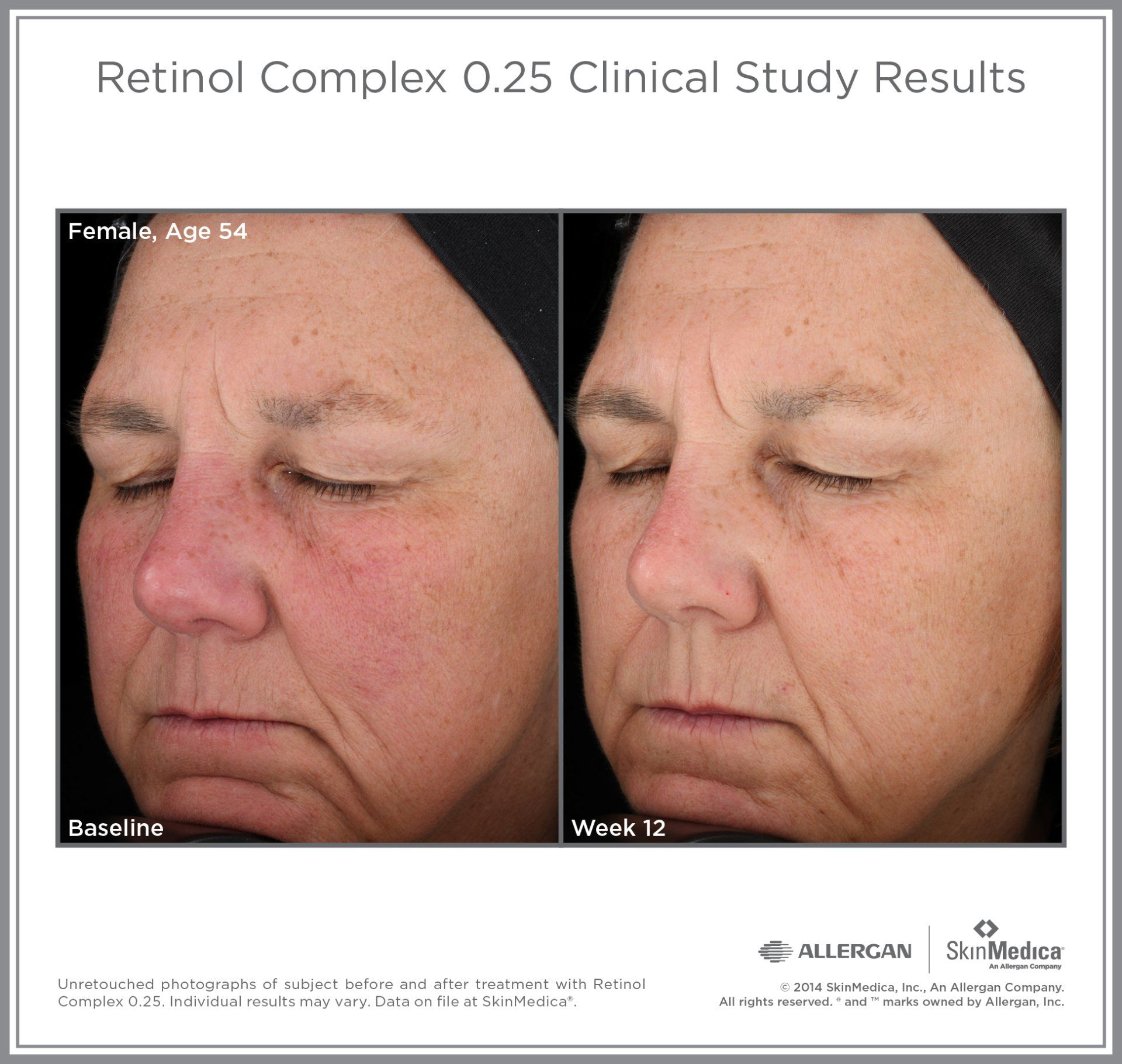
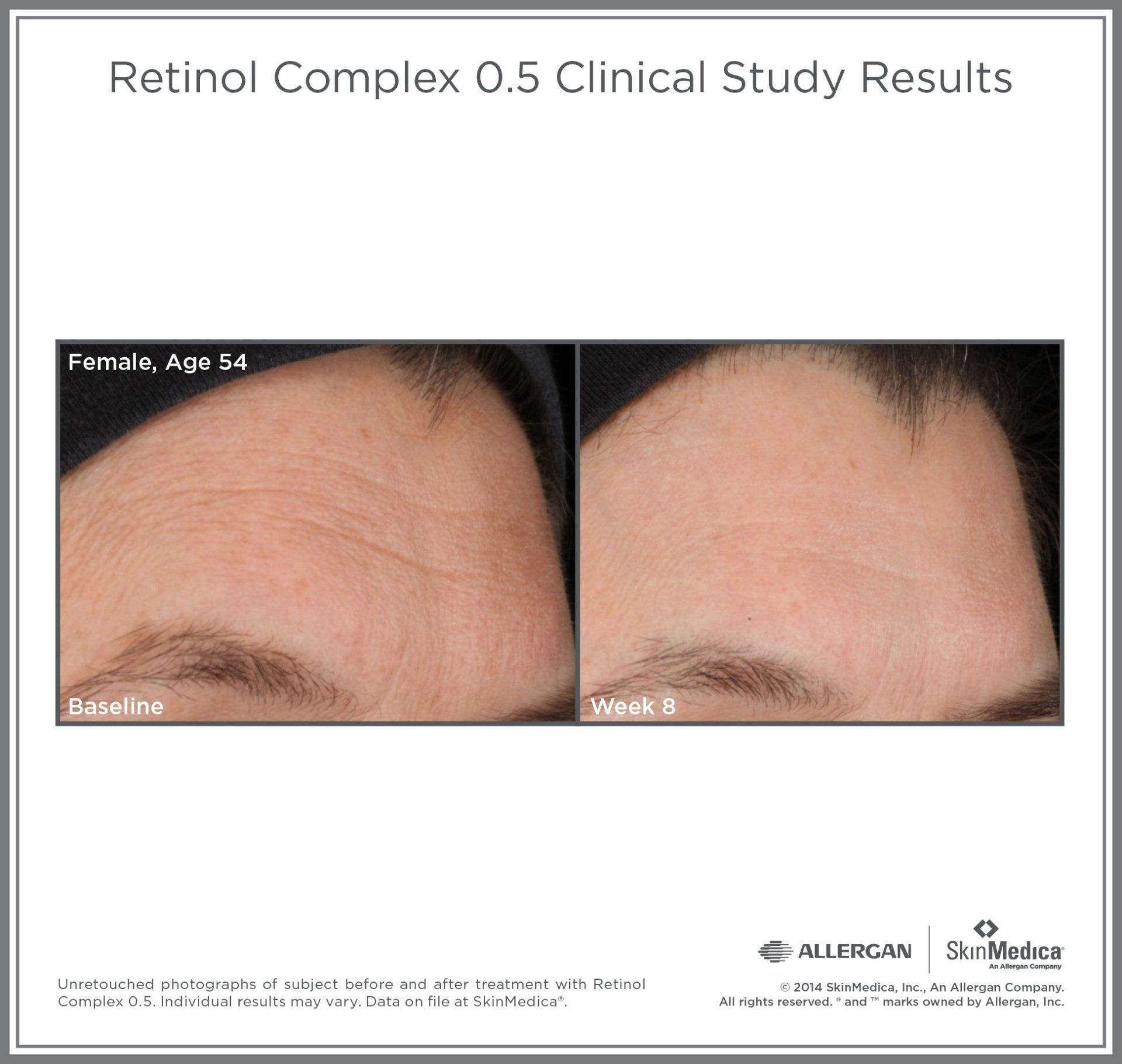
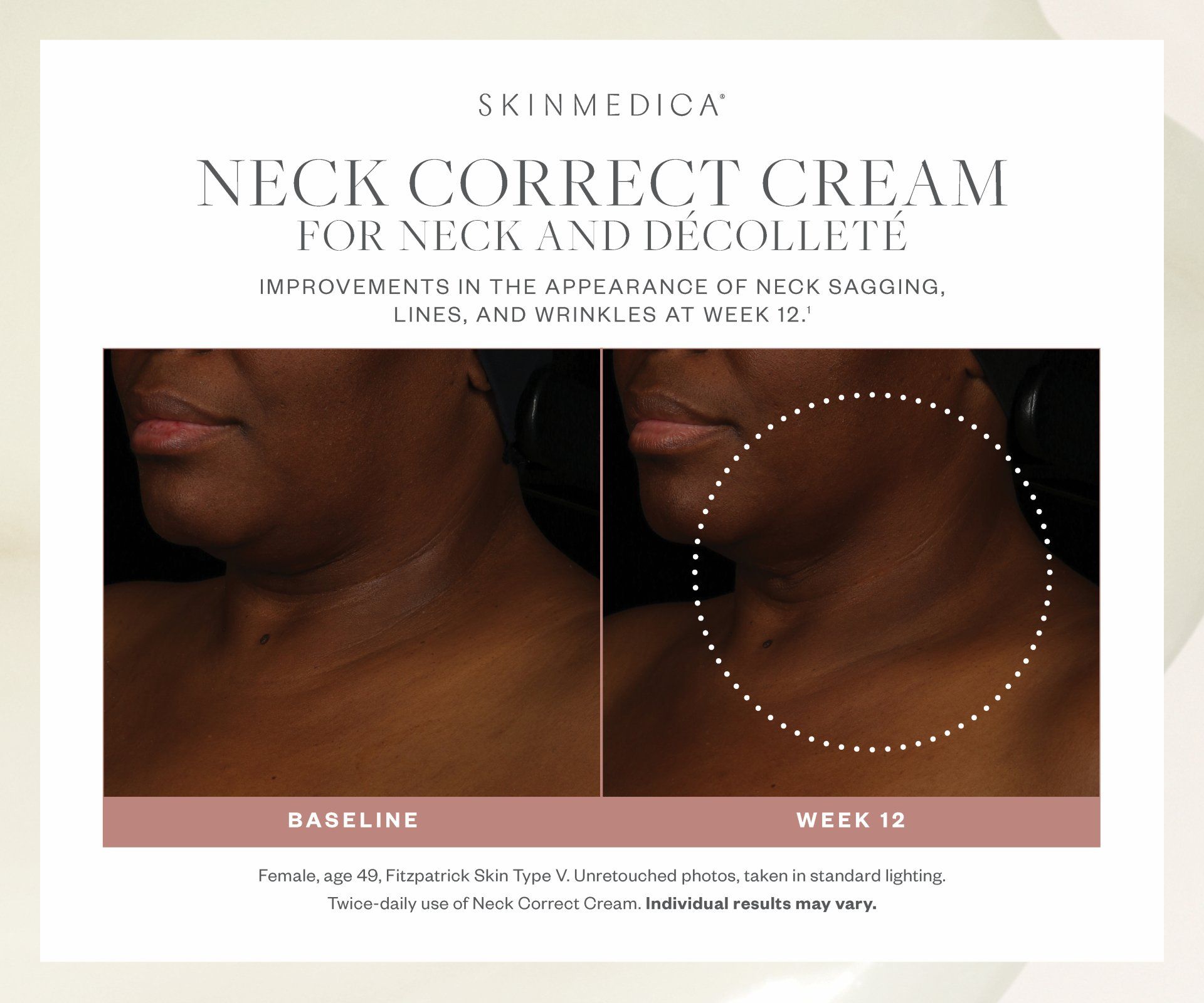
Most SkinMedica® products are intended to meet the FDA's definition of a cosmetic product, an article applied to the human body to cleanse, beautify, promote attractiveness, and alter appearances. These SkinMedica® products are not intended to be drug products that diagnose, treat, cure, or prevent any disease or condition. These products have not been approved by the FDA and the statements have not been evaluated by the FDA.
SkinMedica® Total Defense + Repair Broad Spectrum Sunscreens (SPF 34, SPF 34 Tinted, and SPF 50+) and Essential Defense Broad Spectrum Sunscreens (Everyday Clear SPF 47, Mineral Shield Tinted SPF 32, and Mineral Shield SPF 35) are over -the-counter drug products which are formulated and marketed pursuant to FDA’s governing regulations set forth at 21 C.F.R. Part 352.
The PA rating System is used in Japan to classify UVA protection and is not an FDA requirement on sunscreens sold in the U.S.
SkinMedica® Purifying Foaming Wash is an over-the-counter drug product which is formulated and marketed pursuant to FDA’s governing regulations set forth at 21 C.F.R. Part 333 Subpart D.
SkinMedica® Pro-Infusion Serums Disclaimer
SkinMedica® Pro-Infusion Serums are intended to meet the FDA’s definition of a cosmetic product, an article applied to the human body to cleanse, beautify, promote attractiveness, and alter appearances. These products are not intended to be drugs that diagnose, treat, cure, or prevent any disease or condition. These products have not been approved by the FDA and the statements have not been evaluated by the FDA.