Blog
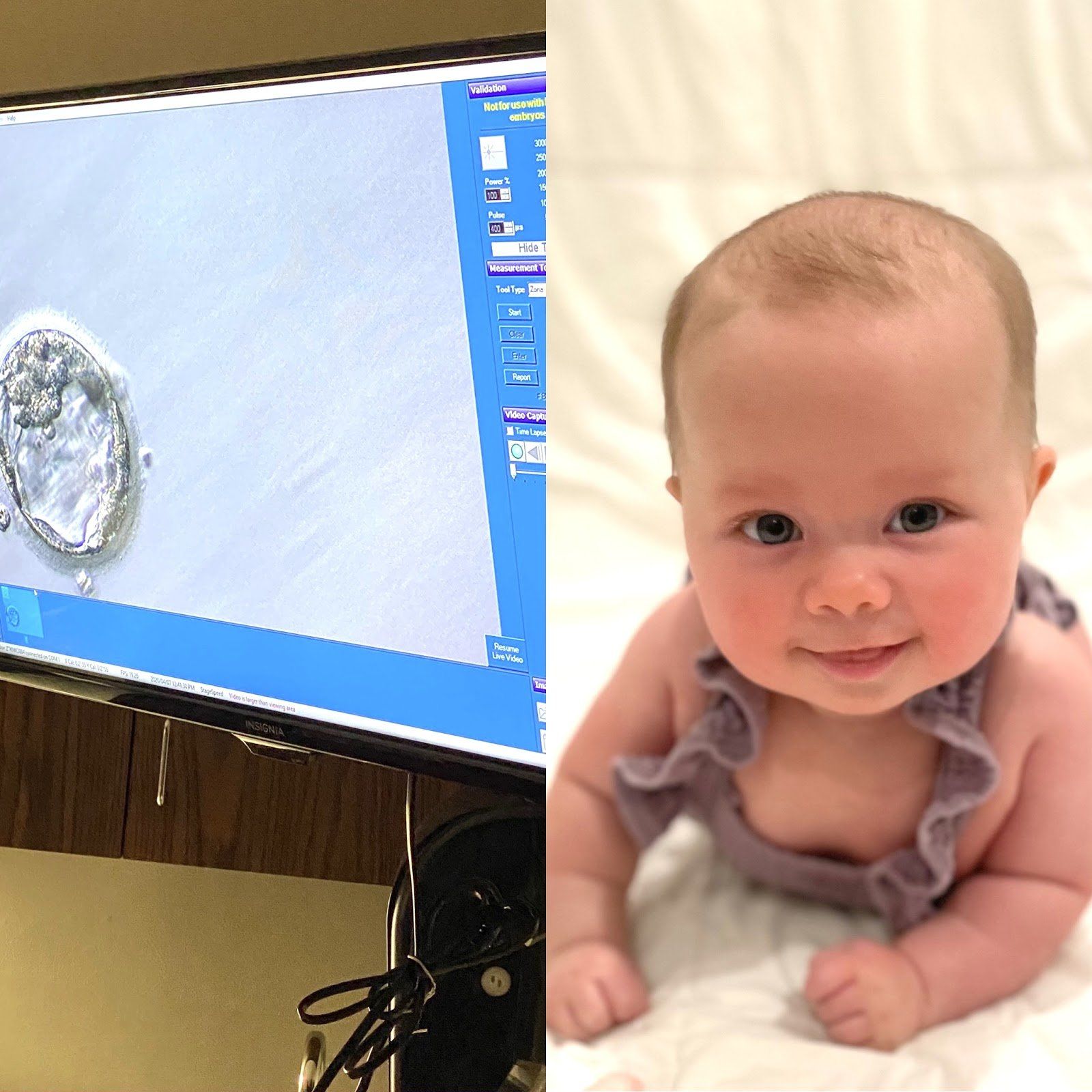
It is " Infertility Awareness Week ." Does it surprise you to know that 1 in 8 couples are inflicted by some sort of infertility? You will notice throughout my story, I may use words different than what you are used to hearing. I will not say 1 in 8 "struggle", because that implies that if we fought just a little harder, struggled against it a little longer, we could change the outcome- which is obviously not the case. Infertility is not a "struggle," it is not a "fight," it is a medical problem that unfortunately afflicts many of us. Well-meaning friends and family may say "it will happen when you least expect it," or, "as soon as you stop trying you'll get pregnant." Well, quite honestly that is not true. Infertility is not something that can be changed by willpower, it is a medical condition that needs to be treated. Sometimes the cause of infertility can be pinpointed- I, for example, have one fallopian tube that is closed. But often there is no definitive reason. The conception of a child requires the combination of so many things to be perfectly in sync- it is no wonder so many couples cannot conceive! Hormones, endometrial health, ovarian health, egg count and age, and semen- all of this has to be functioning properly at the same time to allow a couple to become pregnant when they want. I will tell you our story. Thankfully, it has a happy ending. I met my husband, David, when I was 25 and got married at 27. At the time I was an anesthesiology resident at Georgetown University. We did not try for children right away. Working 80 hours a week and having a newborn did not feel feasible or enjoyable to me. And what little time off we did have we enjoyed going out and experiencing DC or traveling as much as we could afford. Fast forward a few years, I am now 30 and we have decided we want to start our family. Being a physician I knew it could take some time. I was not stressed the first month, or two, but after 6, 7, 8 months… I was both frustrated and concerned. Time seems to slip by when you are counting it in 4 week "cycles" tracking your period and peeing on a stick most mornings. Any couple who has actively tried to get pregnant knows the roller coaster: each month waiting to get the signal you're fertile, trying to conceive, hoping it worked, and then: either it does or it doesn't. And if it doesn't you are deflated but you know that next month- that could be it, that could be the month that it finally works! So you start all over again. Up and down, up and down. Please know, you are not alone on this roller coaster. Hopefully you have a supportive partner with you on the ride. But also, there are millions of women out there doing the same thing. If you are open about your experience you will likely find other women close to you that are on the ride with you. I will spare you all the details but we saw Elizabeth Irby, Women4Women CRNP, and did all the testing for myself and my husband. I do have a closed tube, but you should be able to get pregnant with just one tube so it is still partially unexplained. We tried fertility medication for a few months before deciding to move on to an infertility clinic. After conversation with our fertility specialist, Dr. Long, we opted to go forward with IVF using the invocelle. The invocelle is an awesome little device that allows you to incubate your embryos. After the eggs are fertilized, they are placed in a culture medium in the device and then inserted in the vagina where you incubate them for 5 days. Our first round we were so hopeful, we fertilized 3 good looking eggs. We went back after 5 days, and none of them had developed into usable embryos. I was not prepared for the level of heartbreak I felt at that moment. It was just a bunch of cells, right? But all of a sudden those 3 potential babies were gone in an instant from a glimpse at a microscope. And that was hard. My friends and family stepped up in a big way. I got flowers and well wishes and it bolstered us to try again. The second round of IVF worked. There is still a spot on my butt that is tender from all the progesterone injections, but I wouldn’t trade that for the world. Because our daughter, Rose, is now 4 months old and the most awesome part of our lives. She has a big gummy grin and chuckles like an old man and will completely melt your heart. Thankfully, I have had family and friends who were invested in this process with us. David and I made a conscious decision from the start to be open with those around us about what we were going through. You will be surprised when you start telling people what you are dealing with how many have their own story to contribute. We have made friendships built on the shared experience of infertility. I am glad we chose not to go through it alone. If you have not felt comfortable sharing with your friends and family, ask yourself why? Women have trouble recognizing that this is a disease inflicted upon them, not a failure. I have had the thought "if only I hadn't put off trying to get pregnant until after residency maybe we wouldn't have had to do IVF." But, maybe we would have? I will never know and guess what- it doesn't matter. There is no use thinking the "what ifs," or blaming yourself. I have never once blamed myself for having asthma, and I refuse to blame myself for having infertility. It is not your fault, it is not your body's fault. And that is why I believe Infertility Awareness Week is so important. I hope these stories empower women to be more open with their friends and families. You will be surprised- they want to be there for you, let them.

Most of us have heard of endometriosis at some point. Maybe you know a friend, a relative, or a coworker who has this diagnosis. However, there are a lot of misconceptions about what endometriosis actually is and how it affects patients. Endometriosis is a common, benign disorder that is estimated to affect approximately 10% of women. Endometriosis refers to a condition in which endometrial cells which normally line the uterine cavity are found outside of the uterine cavity. This disorder can come with a variety of symptoms or may be completely asymptomatic. Myth #1: Endometriosis just means you have very painful periods. It is true that the most common presenting symptom of endometriosis is painful periods (the medical term is dysmenorrhea). But as I’m sure many of you know, there are a number of conditions that can cause painful periods, such as uterine fibroids, polyps, ovarian cysts or masses, adhesions, or other disorders. In order to make a diagnosis of endometriosis, we first have to rule out all of these other conditions, which can often be challenging. One major challenge is that endometriosis typically cannot be seen with ultrasound or other imaging techniques. The gold standard for diagnosing this disorder is by performing a diagnostic surgery and actually visualizing or taking a biopsy of the lesions. So diagnosing endometriosis as the cause for painful periods is a lot more complicated than you might think! Myth #2: The more severe your symptoms, the more advanced the disease. As I just mentioned, some women with endometriosis have no pain at all and some have incapacitating pain. It stands to reason that people would believe that this reflects the degree of endometriosis they have. However, some of the worst cases of endometriosis I have seen have been in women who had no symptoms at all! Meanwhile, many of the patients with the worst pain have had only a couple small spots of endometriosis called “implants” when I did a laparoscopic surgery to look. The reason for this is not well known, but it is thought to be largely dependent upon the location of the implants and the degree of new nerve growth that has taken place around the implants. Myth #3: Endometriosis causes infertility. Yes, it is true that endometriosis is present in a large portion of women with infertility issues. One reason for this could be endometriosis on the fallopian tubes that distort or block the tubes. In reality, this is rarely the finding that we see during surgery. There are other theories, such as the presence of inflammation produced by the endometriosis implants, or somehow lowering a patient's reserve of good eggs. While fertility appears to be decreased in many women with endometriosis, it does not necessarily cause the need for IVF (in vitro fertilization). Myth #4: Endometriosis is caused by backward flow of period blood out of the fallopian tubes and into the abdominal cavity. While this is the prevailing theory about how endometriosis occurs, there are several other theories out there. In fact, this cannot be the only way that endometriosis occurs. If that is the only way it happens, then how has it ended up in lung tissue? It has also been found in girls as young as 8 years old who have not yet started menstruating. In fact, it has even been found in women who were born without a uterus! Myth #5: There is no effective treatment for endometriosis. This is simply not true. These implants are estrogen-dependent, which is the reason that the menstrual cycle stimulates their activity. However, this also means that they can be responsive to hormonal therapies. There are many different hormonal and hormone receptor treatments for endometriosis. While these therapies keep symptoms of endometriosis at bay, they do not cure the disorder. When these treatments are discontinued, symptoms often resume. Myth #6: The cure for endometriosis is hysterectomy. Removing the uterus alone often does not cure endometriosis. The ovaries must be removed as well, along with any endometriosis implants. Even when this surgery takes place, a small number of women will still have recurrence of some symptoms if some small implants remain. Myth #7: Endometriosis increases your risk for endometrial cancer. There is no genetic trait associated with endometriosis that has been shown to lead to cancer. There are some very rare types of ovarian cancers – clear cell and endometrioid cancer – that seem to be more common in women with endometriosis, but even with these rare cancers the risk is less than 1%. Therefore, there is no recommendation for increased cancer screening for women with endometriosis Allison Warren, MD

The past year has brought a lot of uncertainty to our lives. Given that pregnancy is already an uncertain time, the unknown surrounding COVID and the COVID vaccine can understandably lead to anxiety. Should you get this vaccine or not? Is the vaccine safe? With this blog, we hope to be able to shed some light on that question. When patients walk into our office, the first question that they often have regarding the vaccine is, “How does this vaccine work?” The COVID vaccines are mRNA vaccines. Because these vaccines are the first mRNA vaccines on the market, there are a lot of questions regarding how they work. The vaccine contains a small amount of genetic material called mRNA. The best way to think about mRNA is as a recipe or as an instruction manual. Once you get the vaccine, the mRNA is taken up by your cells and acts as an instruction manual for your cells to make spike protein, a protein that is found on the COVID virus. Once your cells make the spike proteins, they present them to your immune cells, which then make antibodies against COVID. So, if you contract COVID, your body has the antibodies ready to go to fight the virus! After the mRNA has done its job, it is quickly degraded by your cells. Therefore, there is no evidence that it crosses the placenta. There are two important things to remember when considering these vaccines. First, because this vaccine does not contain a live COVID virus, it cannot give you COVID. In addition, because the mRNA is never taken into your cell’s nucleus, it does not have the ability to change your DNA. This new technology is very exciting because mRNA vaccines give us a much more efficient way to produce vaccines than previous vaccine technology using live or inactivated virus. There have been two vaccines that have been approved for emergency use by the FDA. These vaccines have been produced by Pfizer and Moderna. The Pfizer vaccine is given in two doses 21 days apart and has been approved for use in people ages 16 and older. In the trials, the Pfizer vaccine was found to be 95% effective. To put that in perspective, consider that according to the CDC, the 2019-2020 flu vaccine was 39% effective. The Moderna Vaccine has been approved for people aged 18 and older. It is given in 2 doses 28 days apart and was found to be 94.5% effective in trials. Once you start a vaccine series, you should complete the series using the same manufacturer. Both doses in the series are necessary to receive full protection. If more than 21 or 28 days have passed since your first vaccination, the second dose should be given as soon as possible, but no doses need to be repeated. The natural next question from our patients has been, “Is it safe?” Unfortunately, pregnant women were not included in the COVID vaccine trials. There are further ongoing studies, but the safety data is not yet available. However, the American College of Ob/Gyns, the American Society of Reproductive Medicine, and the Society for Maternal-Fetal Medicine all recommend that women who are trying to conceive, pregnant women and lactating women who are eligible for the vaccine should be offered the vaccine, even if they have previously had COVID. In addition, the current guidelines from these societies do not recommend waiting a specific amount of time to try to conceive after vaccination. But, what does that mean for you? We know that pregnant women are at risk of having more severe disease if they contract COVID-19. Many reports have shown that pregnancy women are 3x more likely to need an ICU admission compared to non-pregnant women if they contract COVID. Depending on your health history and other underlying conditions, your risk of severe disease may be even higher. Thus, when deciding whether or not to receive the vaccine, we must consider four factors: 1) the level of activity of the virus in the community 2) the potential efficacy of the vaccine 3) the risk and potential severity of maternal disease, including the effects of disease on the fetus and newborn and 4) the safety of the vaccine for the pregnant patient and the fetus. Unfortunately, the level of activity in our community is currently high, and we know that both vaccines have about a 95% efficacy rate. Thus, a lot of the decision is likely based on the potential risk of maternal disease and the safety of the vaccine for the pregnant patient. It is important to recognize that because the vaccine does not contain live virus, it is not thought to cause an increase in infertility, miscarriage, stillbirth or congenital abnormalities. Therefore, your individual factors as a patient will often dictate whether or not you receive the vaccine. What are your underlying health conditions that increase your risk of having severe disease? What is your risk of contracting the disease? We recognize that the risk of someone who works from home may be different than the risk of someone who works in healthcare. If you decide to receive the vaccine during pregnancy, when is the best time to get it? At this time, there is not a recommendation for optimal timing of vaccine administration in pregnancy. However, the COVID vaccine should not be given within 14 days of another vaccine, which should be taken into consideration when giving other vaccines in pregnancy. Thus, the influenza and Tdap vaccine should not be given within 14 days of the COVID vaccine. When taking all of these factors into consideration, we encourage you to talk to your provider at your next appointment about whether or not to receive the COVID vaccines and when to receive the vaccine. There is so much individualization that goes into this decision. We know that this is a personal decision, and we encourage you to ask questions and let us help guide you in your decision making. In the meantime, keep up the handwashing, maintain social distance and mask up! As always, we are here for you regarding any COVID or pregnancy-related questions! Katie Harkess, MD

Dry skin caused by cold weather can be extremely irritating and sensitive. When the skin is dry it may become itchy and flaky. It may also feel rough or appear chapped and have a dull or lifeless appearance. The skin may appear red and cracked causing it to bleed. In extreme circumstances where home remedies or visiting a certified aesthetician does not help with dry skin discomfort, it may be necessary to see a dermatologist. Below are a few tips to help with dry skin during the winter months: Stay hydrated. During the winter months, it’s easy to opt for warm comfort beverages such as coffee, tea, and hot chocolate; however, it is important to drink plenty of water as well! Although it is cold and windy outside, which can exacerbate the symptoms of dry skin, many indoor environments have dry air, which in turn can also make dry skin worse. Washing the face with a non-soapy facial cleanser will help with keeping some of the natural oils in the skin. Avoid over-cleansing with scrubs and abrasive cleansers. During winter months, washing with abrasive cleansers can strip the skin and damage the cells causing cracked, dull, itchy skin. It is better to use a gentle cleanser. Immediately after cleansing, use a creamy moisturizer to hydrate the skin. The skin is softer and the pores are open after washing, thus the skin is able to receive products better. Hyaluronic acid treatments and hydrating serums such as collagen will help to put extra moisture in the skin. Although it’s tempting, avoid extremely hot showers so that the moisture content in the skin will remain balanced. Using moisture masks weekly or bi-weekly will help to keep the skin hydrated and help with the dull or lifeless appearance. I hope you find these tips helpful! Feel free to make an appointment with me for a free consultation! Call 256-759-9269 . Felicia McCarty , Licensed Aesthetician

While a healthy mom and baby are our primary goals in childbirth, the process by which you get there is just as important. Women take different approaches to pregnancy and birth. Some want to be heavily involved in education and decision making. Others do not want the responsibility, trusting their provider to make decisions on their behalf. Regardless of where you fall on that spectrum, my hope is that you feel educated and supported in your wishes. So what exactly is a birth plan? A birth plan is a written document allowing you to communicate your wishes and preferences for your labor/birth experience to your provider and other members of the birth team on delivery day. Not everyone wants a birth plan, but they are beneficial for moms who may have preferences outside of some of the normal/routine procedures done in the hospital. A birth plan can be helpful for those whose provider is not on call when they go into labor as well as for the staff in the hospital. When you are in the midst of labor it can be difficult to discuss your wishes with your birth team so the birth plan comes in handy. So, how do you know what to put in your birth plan? Throughout pregnancy you will learn from your OB provider about the processes of labor and birth. Utilize them to ask questions at each visit because that is YOUR time to educate yourself and discuss goals/wishes for birth. While many women often receive encouragement and advice from friends and family, it’s important to clarify questions with your OB provider. Some things to consider include pain management options, desired mode of birth, labor positions, delivery positions, breastfeeding or formula feeding, etc. You have nine months to ask these questions so try not to get too overwhelmed by that list! Tips on making a birth plan: Keep it short and concise and one page if possible. The hospital staff and your delivering provider will be quite busy on delivery day taking care of you so the quicker they can read it the easier your wishes will be honored. Organize it into categories: labor, delivery, newborn care, cesarean birth, etc. Ultimately, some things in birth are unpredictable and being flexible is important. So, to answer the question, do I need a birth plan? That’s completely up to you. For some, the anxiety and stress of feeling like you need a plan is overwhelming. If this seems like just another task for your list, then maybe a birth plan isn’t for you. But for some people, a birth plan helps ease anxiety and stress around giving birth by allowing them to have a voice in their care and to think about their goals. Regardless of whether you have one written or not, you should feel confident in your care with your provider and trust that they have your best interests in mind regarding your birth. — Tiffany Golub, CNM, CRNP Click here to download our Birth Plan Checklist Template.

August 26 commemorates Women’s Equality Day, a proud day for all American women This year marks 100 years since the passage of the 19th Amendment to the U.S. Constitution, which granted all women the right to vote. Women earned the right to vote through the decades-long struggle of heroes such as Elizabeth Cady Stanton, Susan B. Anthony, Jane Addams, Ida B. Wells, Lucy Burns, and Mary McLeod Bethune who boldly persisted against those who wished to preserve the status quo. (Fun Fact: In 1895, Suffragist Susan B. Anthony came to speak at City Hall in Huntsville!). Women’s Equality Day gives us time to reflect on the work women have done across generations removing barriers, overcoming obstacles, and pursuing new ideas. One of the greatest obstacles women continue to face is limited access to gender-specific health care. Even now, many women report that their health concerns have been discounted in medical settings, often leaving them feeling brushed off or not taken seriously. Today, one in ten women in the US is uninsured and this number is even greater among minorities and women with lower socioeconomic status. Moreover, among the 10 highest income countries worldwide, American women suffer the most chronic illnesses and lead in rates of maternal mortality. Thousands of US women often skip necessary healthcare appointments due to the relatively higher costs of medical care in the US compared to other economically advanced countries. Due to the unequal distribution of healthcare especially in rural areas, sixteen percent of women in Alabama state they have no healthcare provider. In addition, women in our state tend to have higher incidences of diabetes, arthritis, asthma, COPD, and kidney disease compared to men. Alabama women also have a higher likelihood of poor mental health at 27% compared at 16% for men. As we women continue to outlive our male counterparts, we will need to have better access to health providers as well as more gender specific approaches to healthcare. As we celebrate Women’s Equality Day let’s not forget that we are still not “there” yet. Let’s advocate for ourselves and each other in every way. I wonder what our sisters who helped pave the way 100 years ago would think about where we’re at today? I know they would be proud. They understood, like we do, that EVERY woman is worthy of healthcare that focuses on her unique needs as a female. It was true then what is true now; EVERY woman is worth it. Until next time, Elizabeth Irby CRNP

There was a time, not so long ago, when women had to remember to take their birth control pills every single day! Or write down when it was time to change their NuvaRing or birth control patch- sometimes making a secret mark on their calendar (known only to them) to remind them! Well, that is history. ICYMI, the App Store is bursting with apps to help the women of today take charge of not only their birth control, but tracking their cycles, side effects and moods. A quick search of “Birth Control Apps” today yielded dozens of different apps. Many have cute, lady-like names such as Eve, Missy, Tia and Mia. “Flo” gets points in my book for the double meaning. Two of the most popular apps are “My Pill” and “Birth Control Pill Reminder.” Both are highly rated by users. They send you texts to remind you (discreetly) to take your pill, remove or place your NuvaRing or replace your patch. Both include reminders for refills when you are getting low, have places to take notes and track symptoms, and even an ability to “snooze” the reminder and have it pop up again later in the day! There are several apps for tracking natural cycles if you are doing natural family planning as opposed to prescription birth control. The “Natural Cycles-Birth Control” app is rated 4.8 and has 5,200 reviews. It can also be used as a fertility tracker if you are trying to get pregnant. You can delve even deeper into women’s health the more you scroll through the available apps. You will see health journals and apps that have virtual access to a provider for a fee. Even ones that promise to help your sex life. For those of you married to your devices and on birth control, I am sure you have already searched for these apps before reaching the end of this blog – if you aren’t already using one! They are a convenient way to make sure you don’t get off track and have break-through bleeding or an unplanned pregnancy. — Dr. Anne Marie Reidy

Fear about an unknown disease can be overwhelming and stressful as we face uncertain times. You may be worried about finances, your health or the health of your loved ones, job security or juggling parenting while working from home. These are very normal stressors. Our bodies can respond to stress in a variety of ways. Below are some physical responses to stress: Headaches Increased or decreased appetite Changes in sleep patterns Muscle tension Fatigue Increased tobacco, alcohol or drug use In addition to physical symptoms, you may experience some changes to your mental health which may include: Feeling isolated Feeling down or discouraged Feeling helpless Feeling out of control Increased emotional outbursts While people handle stress differently, it is important to learn coping strategies and stress management skills to keep you and your family strong both mentally and physically. While caring for your family is important, it is also important to make yourself a priority. The Mayo Clinic lists several ways to promote self-care during this pandemic. Click here for a full list. Here are some of the ways to keep yourself healthy: Fuel your body by eating as cleanly as possible. This includes lean meats, lots of fruit and vegetables and plenty of water. Try to limit sweets and junk food. Try to keep a regular routine and sleep schedule. Aim for at least 8 hours of sleep per night and try to go to sleep and wake up at the same time every day (including weekends). Strive to exercise at least 30 minutes per day most days of the week. Do an activity you enjoy like walking, biking or jogging. “Enjoy” is the key word! Take a break from the news and/or social media. Hearing about COVID-19 constantly can be upsetting. Avoid risky behavior by limiting alcohol intake and avoiding drugs. If you are a smoker, it can be difficult to quit during times of stress, however, decreasing even a small amount of tobacco products can be beneficial. As far as alcohol intake, the CDC recommends that women of legal drinking age should have no more than 1 alcoholic beverage per day. Try to stay connected to family and friends. Use technology to check in regularly. Schedule check in days. This will give you something to look forward to! Lean on your faith or personal beliefs. Talk to fellow believers for support. Try a new hobby. Have you wanted to try some new recipes or learn to crochet? Now is a great time to make that happen! Take some quiet time to pray, meditate, read a book or take a hot bath. If you have kids, maybe ask your husband or partner to take the kids for a walk or bike ride while you have some quiet time. Spend some time outdoors. Fresh air is good for the soul. Just make sure you maintain social distancing guidelines. Try to help your community. Donate to a food bank. Reach out to elderly neighbors. Look for ways to help others when you are able to. If you are feeling overwhelmed – ask for help or talk to someone. No one knows your inner thoughts but you. Reach out to your medical provider, counselor or someone you trust. As a women’s health provider in North Alabama, I am seeing more and more patients having increased depression or anxiety during this pandemic. And that’s completely understandable. It takes courage to ask for help and to admit when you are struggling. Accepting help is a sign of strength. We at Women4Women OBGYN are here to help you any way that we can. If that means starting or increasing a medication, referring you to a counselor, or just to have us as a listening ear; we are here for you. Our wish for every woman is to stay healthy, stay hopeful and stay strong. *Note: If you have any thoughts of harming yourself or someone else, please contact: Crisis Services of North Alabama 256-716-1000 National Suicide Hotline 800-273-TALK Or Call 911 Until next time, Elizabeth Irby CRNP


